BRUSSELS, 5-Sep-2023 — /EPR HEALTHCARE NEWS/ — The Lancet Regional Health – Europe journal has published a scientific paper on 7 August 2023 authored by a distinguished team of international researchers as part of their work in the IDAlert project. Titled “Decision-Support Tools to Build Climate Resilience Against Emerging Infectious Diseases in Europe and Beyond“, this paper introduces a transformative approach to tackle the emergence and transmission of climate-sensitive infectious diseases in Europe, informing cross-sectoral policy while improving the long-term climate resilience of health systems to infectious disease risks.
Climate change is one of several drivers of recurrent outbreaks and geographical range expansion of infectious diseases in Europe. This paper proposes a collaborative approach to develop policy-relevant indicators and decision-support tools. These tools are designed to comprehensively track and anticipate climate-induced disease risks across various domains, including environmental hazard, exposure patterns, and vulnerability factors. With a keen focus on the interconnectedness of animals, humans, and the environment, the framework promises a holistic perspective to address this multifaceted challenge.
The lead author and IDAlert project coordinator Joacim Rocklöv highlighted, “Our decision-support tools offer a multi-dimensional perspective that transcends traditional silos. By examining the nexus of animals, humans, and the environment, we’re unlocking a more comprehensive understanding of disease dynamics a pre-requisite for more timely and effective outbreak preparedness.â€
The heart of this novel framework lies in the co-production of early warning and response systems with stakeholders and end-users, as well as tailored tools to assess the costs and benefits associated with climate adaptation and mitigation strategies across diverse sectors. By fostering greater resilience within regional and local health systems, the framework aims to strengthen Europe’s capacity to respond to health crises, even in the face of changing environmental conditions.
As part of its approach, the IDAlert project will integrate multi-level engagement, innovative methodologies, and novel data streams, and tap into locally generated intelligence and empirical insights through case studies. This strategy empowers experts to quantify the effects of climate-induced disease threats in areas undergoing rapid urban transformation and contending with heterogeneous health risks. The ultimate aspiration is to bridge the gap between knowledge and action, delivering an unparalleled integrated One Health—Climate Risk framework that will empower policymakers, healthcare professionals, and communities to mitigate risks and bolster resilience.
About The Lancet Regional Health – Europe Journal:
The Lancet Regional Health – Europe Journal is a prestigious journal renowned for publishing groundbreaking research on health challenges worldwide. It aims to promote the advancement of the research agenda, clinical practice and health policy in Europe with the goal of improving health outcomes for all people regionally and globally.
About the IDAlert project:
IDAlert – Infectious Disease decision-support tools and Alert systems to build climate Resilience to emerging health Threats – officially started on 1 June 2022 is a € 9.18 million project and lasts for five years. The project is funded by the European Commission under the Horizon Europe programme with Grant Agreement number 101057554. More information: www.idalertproject.eu
Note to Editors:
For more information about the paper or to request an interview with the researchers, please contact the IDAlert project: contact@idalertproject.eu.
The full paper, “Decision-Support Tools to Build Climate Resilience Against Emerging Infectious Diseases in Europe and Beyond,†is available on the Lancet Regional Health Journal website:
https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(23)00120-5/fulltext
SOURCE: EuropaWire


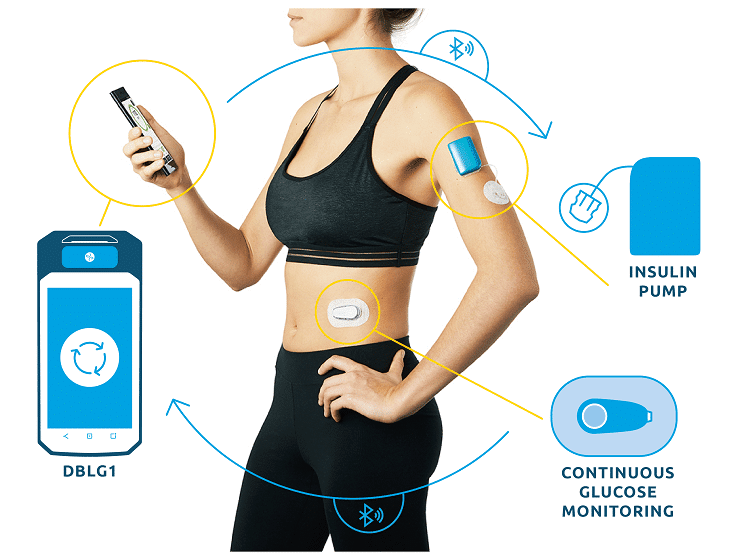

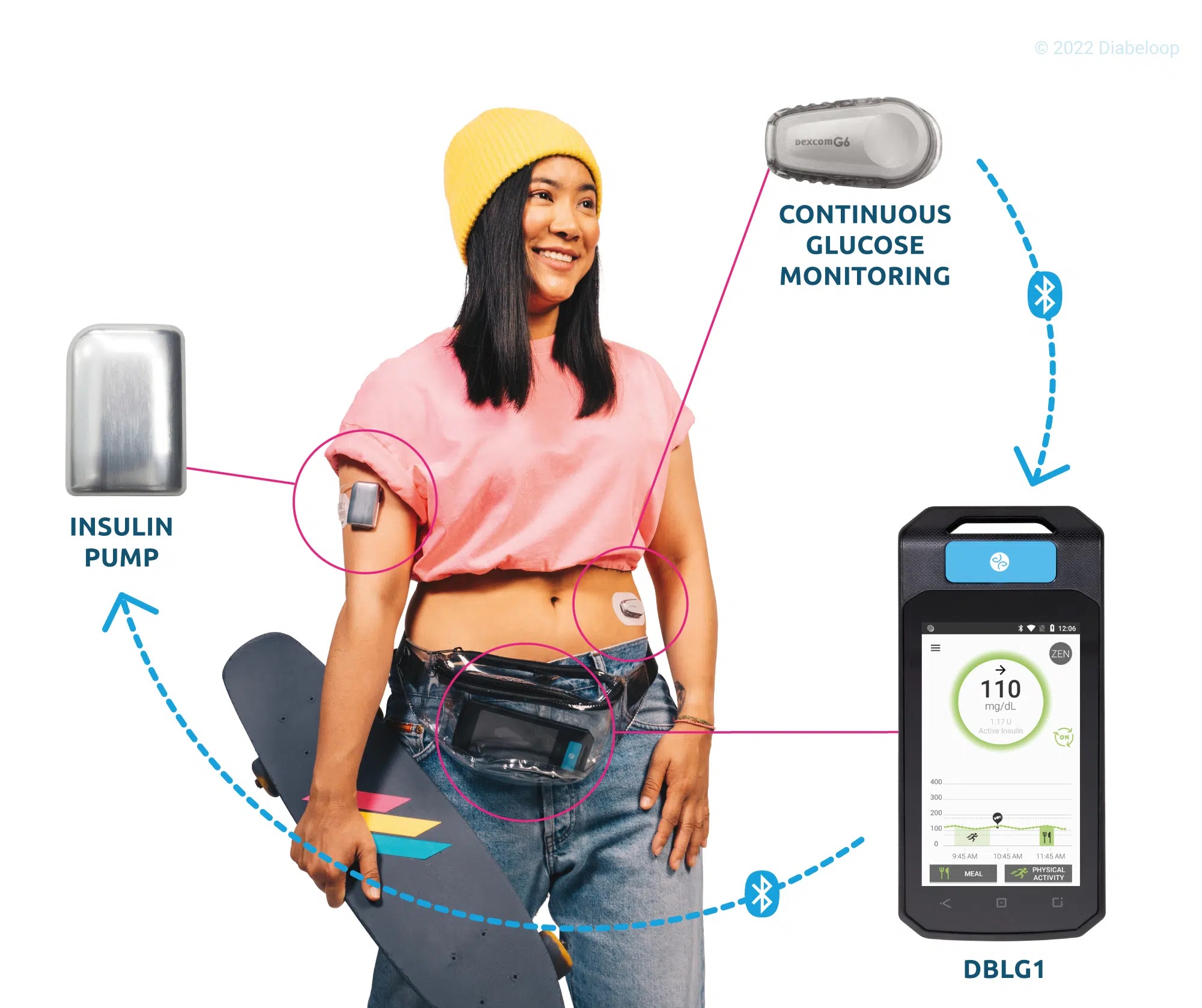
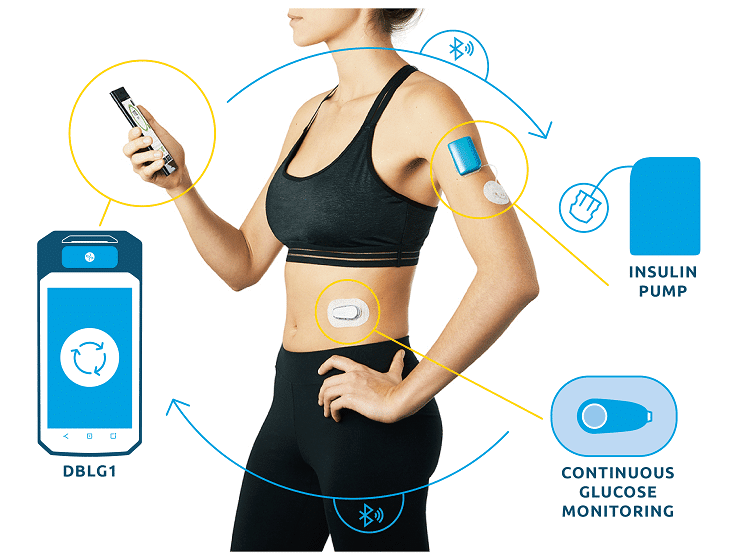
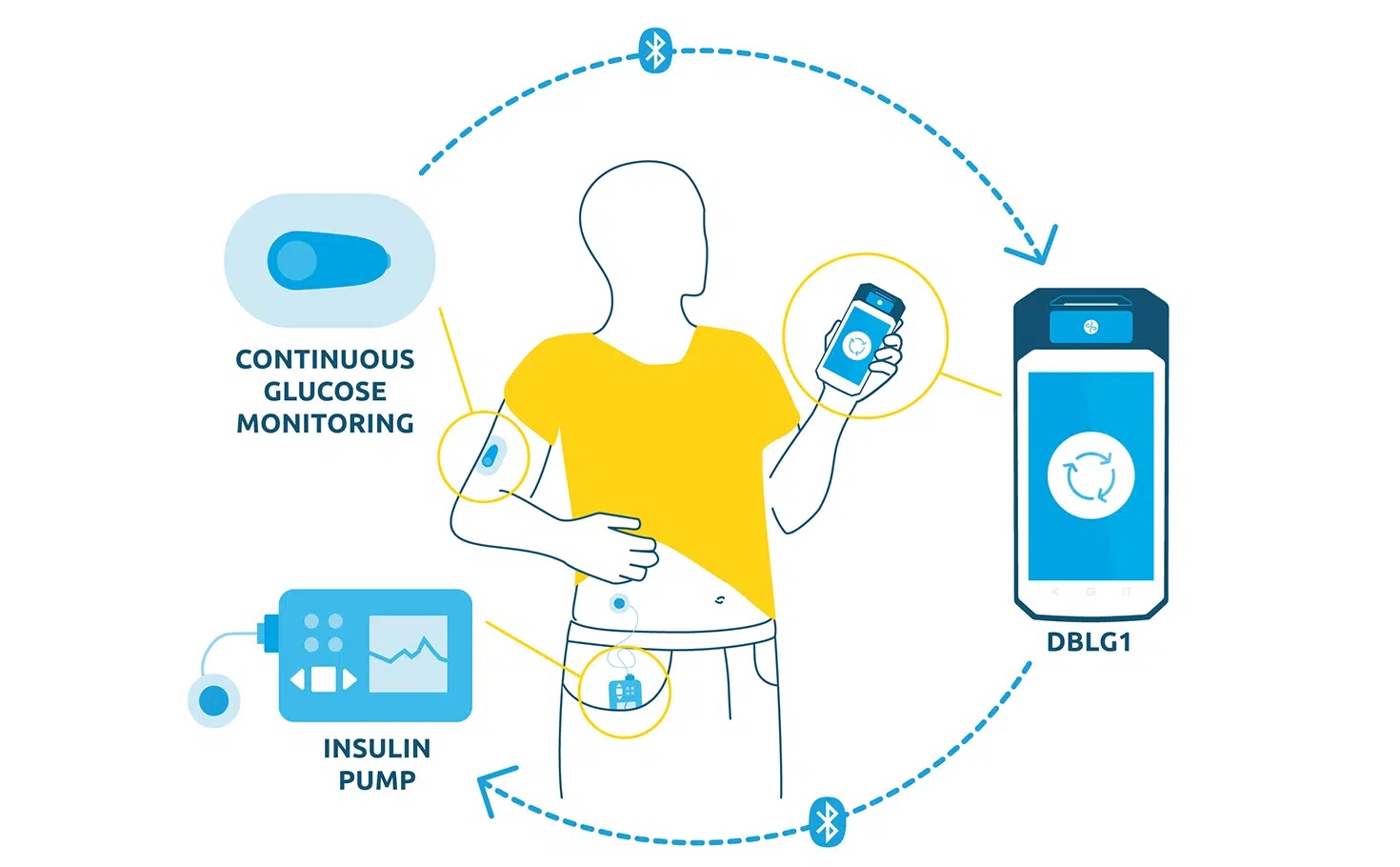
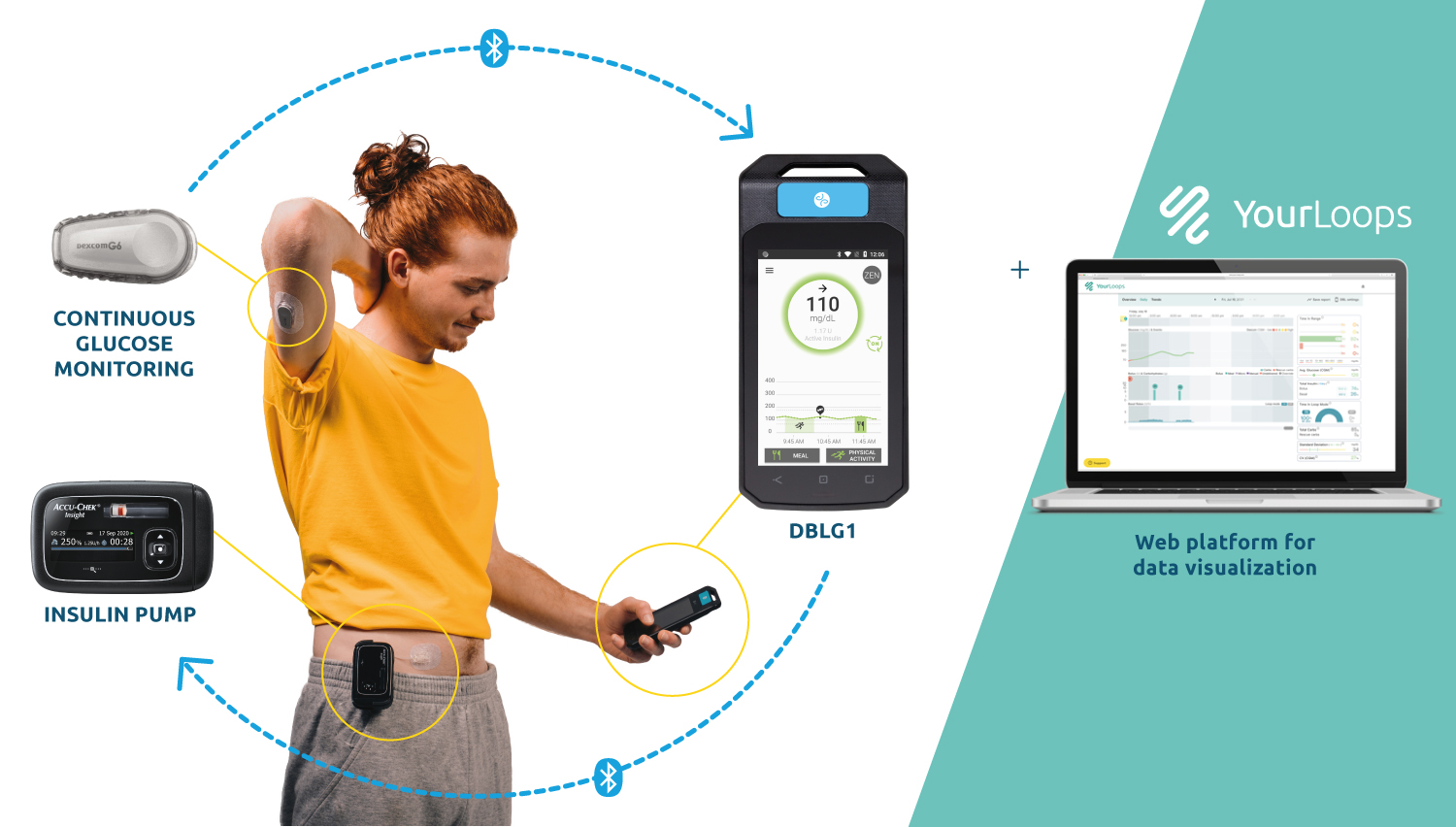
 Listen to all the testimonials in the first episode of the exclusive podcast Diabeloop Stories on Spotify :Â
Listen to all the testimonials in the first episode of the exclusive podcast Diabeloop Stories on Spotify :Â