WARSAW, 13-Nov-2023 — /EPR HEALTHCARE NEWS/ — IQ Biozoom, a medical technology startup focusing on developing non-invasive home diagnostics, is set to make non-invasive home testing more convenient with their innovative technology that allows for the possibility to measure the concentration of biochemical substances in liquid analytes such as saliva, sweat, urine or tears. More people around the world will be empowered to monitor the course of various diseases from the comfort of their own home with laboratory precision using saliva analysis. Currently,  non-invasive home tests measuring glucose and lactate levels in saliva and, in the near future, hormones or CRP proteins, are being developed as part of the technology development.
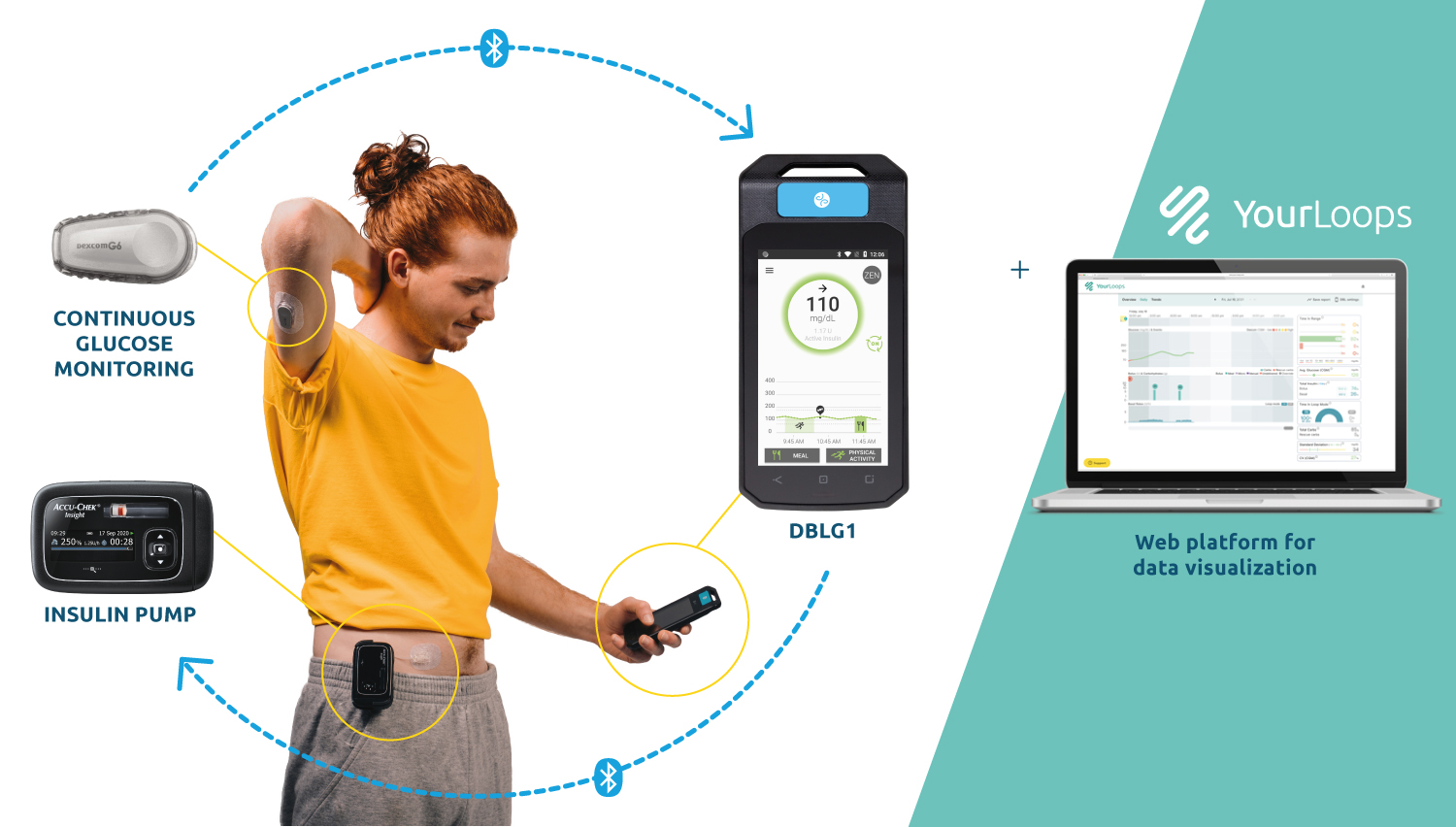
IQ Biozoom’s technology includes a sophisticated biosensor system that integrates inkjet printing technology with advanced semiconductors, specifically thin-film transistors. Although not yet in commercial use, it allows the concentration of a selected biomarker to be determined based on contact between a disposable test strip and a body fluid such as saliva. According to analyses, when measuring glucose concentration in saliva, IQ Biozoom’s cutting-edge technology is up to ten times more accurate than current market standards. This means that even very low concentrations of selected biomarkers in the analyte can be determined with laboratory precision. This can be particularly relevant in those cases where the physiological concentration of a selected substance is very low, or where the mere presence of a biomarker in an analyte is indicative of a health disorder.
Most current blood glucose meters need a finger prick which is usually uncomfortable for patients. IQ Biozoom’s technology enables biomarker levels to be tested with laboratory precision based on saliva analysis, making it a completely non-invasive and painless method.
The solution will enable people with, for example, metabolic and endocrine disorders, to monitor their health and manage their disease, as well as take appropriate preventive care. The devices being developed based on IQ Biozoom’s technology are intended to be used for self-contained, non-invasive home diagnostics, allowing for precise laboratory-standard results.
Monitoring biomarkers and analysing their data retrospectively can help to select the right therapy for a patient. By monitoring biomarkers before, during and after therapy, it is possible to assess whether a patient is responding to treatment. If biomarker levels improve, this may indicate that the therapies are working. If there is no improvement, the therapy may need to be changed. Retrospective analysis of biomarker data can help to personalise therapy, tailoring it to the individual patient. The technology also has applications in personalised sports medicine, particularly in the context of physical performance assessment, training planning and training unit selection. Different athletes may have different lactate threshold which means their training plans should be tailored to their individual body characteristics. Testing lactate levels allows for precise individualisation of training.
Currently, invasive glucometers are the devices of choice for glucose level monitoring. According to the Polish Family with Diabetes report, published by the Association for Diabetes Education SED – 84% of people with type 1 diabetes would prefer to use non-invasive glucose monitoring methods, while 60% skip part of their daily testing due to the need for fingertip pricks. In addition to the discomfort felt, the disruption of the skin increases the risk of an infection. In 2021, IQ Biozoom started developing their innovative technology to non-invasively monitor glucose and lactate levels. Other biomarkers such as cortisol or CRP proteins in the body are in the R&D pipeline.
In the third quarter of 2023, IQ Biozoom obtained funding from the VC Link fund, which supports Polish companies developing breakthrough technologies with global potential. But the seed round is not closed yet with the startup seeking further investment to implement the technology and go to market.
The company expects an even faster growth rate in the near future. The home diagnostics market is growing at 5.6% year-to-year and is expected to exceed $13.6 million by 2033, according to FutureMarketInsight.com.
About IQ BIOZOOM
IQ BIOZOOM Ltd (https://iqbiozoom.com/) develops technology for non-invasive home diagnostics. The company is working on innovative devices for measuring the concentration of biochemical substances in saliva: glucose, lactates, and hormones, to enable people with metabolic and endocrine disorders to monitor their health and manage their disease, as well as applying appropriate prevention. IQ Biozoom technology will be designed for home self-testing. In the near future, the company intends to focus on expanding the technology usage to measure the levels of other substances important for diabetics (diabetes package) and woman (hormone package) ‒ in saliva.
SOURCE: EuropaWire